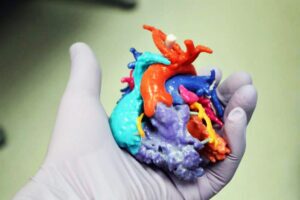
Additive Manufacturing for Medical Applications
The use of additive manufacturing applications is on the rise, with the market value expected to increase from $6 billion in 2017 to nearly $26
The medical industry relies on customized product solutions made to the highest standards. When safeguarding human health is the priority, there can be no compromises in the quality of materials or workmanship for medical manufacturing. In addition, the entire supply chain must demonstrate complete transparency and traceability to ensure no contamination of the finished product. That’s one reason why world-class suppliers of medical manufacturing services like Star Rapid are certified to the ISO 13485 standard.
We’re proud to have worked with industry leaders to bring new products to market in the fields of clinical care, diagnostics, research, and rehabilitation. Our success stories include fitness trackers, health monitors, drug delivery devices, enclosures for medical equipment, prosthetics, and many other healthcare solutions.
Star Rapid supports your design freedom by having no minimum order quantity or value restrictions. We’re experts at providing customized medical prototyping and rapid product development solutions for projects of any size or complexity.
Get to market fast. Contact us today to see how we can help you with our medical manufacturing services.





We make high-precision mold tools for medical plastic injection molding of specialty resins including POM, PEEK, Ultem, and more. Fast turnaround with full material traceability helps you to achieve your regulatory requirements for medical products.

Polyurethane vacuum casting is the ideal medical prototyping process for making high-fidelity copies of plastic cases and components. Minimal tooling investment and short lead times mean that you get a production-quality part quickly and economically.
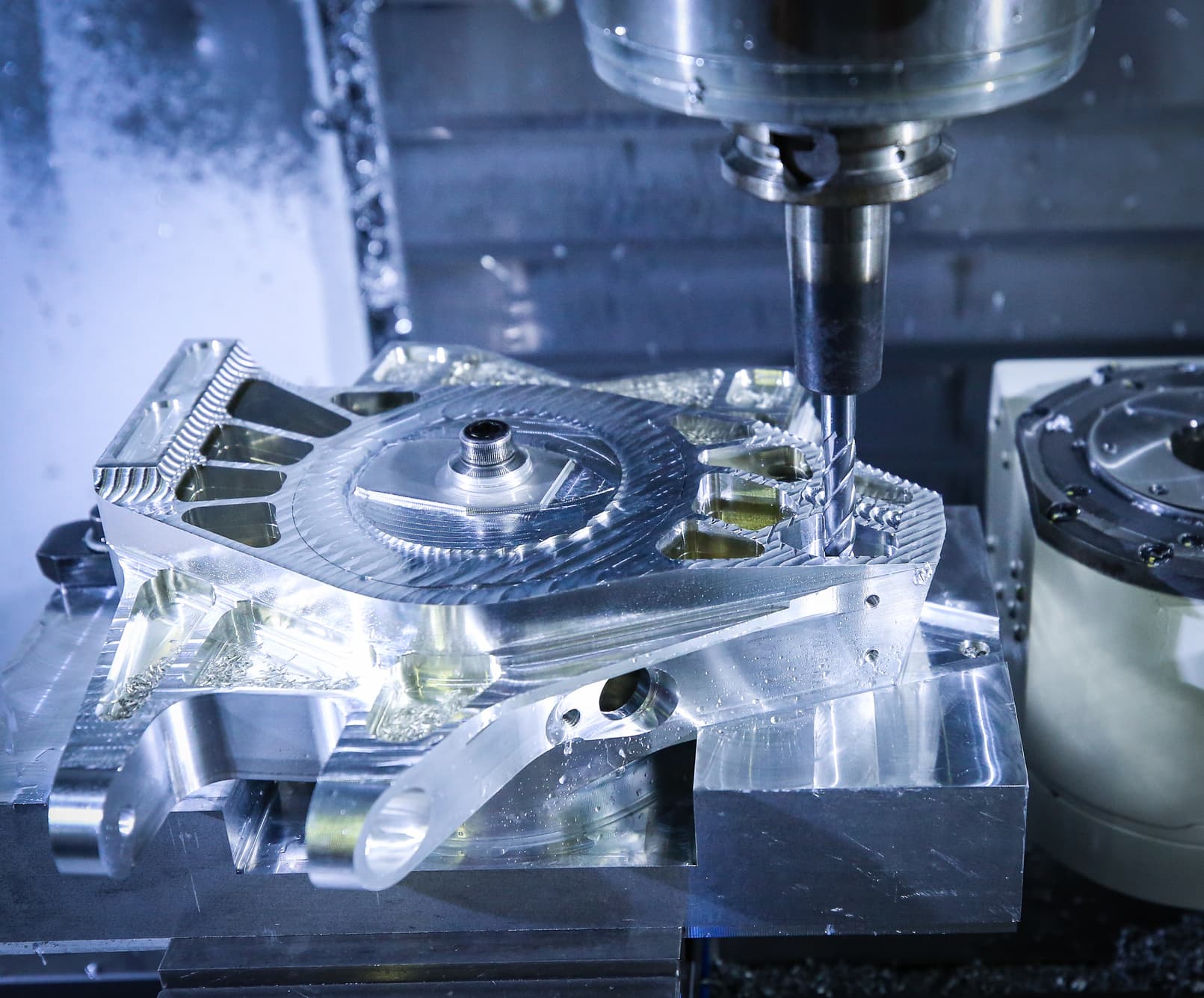
Precision CNC machined parts in unlimited volumes. Complete DFM review helps you to optimize your custom machined parts from a variety of medical-grade metals, including titanium, stainless steel, cobalt chrome, and many copper alloys.
Medical devices fall into several categories, depending on the degree of risk they pose to human health. Devices that can be used inside the body pose the highest risk factor, and therefore, are controlled by the tightest regulations. These include arterial stents, heart valves, surgical instruments, artificial joints, bone grafts, and prosthetics. At medium risk are products that have temporary contact with the skin. These include heart monitors, tongue depressors, IV drips, fitness trackers, and other diagnostic equipment. At the lowest risk is support equipment such as clamps, pill dispensers, trays, clinical furniture, and laboratory testing apparatus.






Ensuring the reliability of the supply chain is critical in the medical industry. That’s why we use advanced metrology equipment to verify all incoming raw materials and will provide you with chemical analysis and full certificates of compliance for your peace of mind. To support the needs of the medical industry, we offer a range of materials including titanium, stainless steel, TPE, POM, PEEK, ABS, nylon, silicone rubber, and more.
Our facility is fully certified to ISO 13485:2016, 9001:2015, 14001:2015, and 45001:2018 standards.

The use of additive manufacturing applications is on the rise, with the market value expected to increase from $6 billion in 2017 to nearly $26
As we all know, devices used in medicine and healthcare must meet the highest standards of performance and reliability. This is as it should be,

What is ISO 13485:2016? ISO 13485:2016 is a quality system management standard. It’s similar to ISO 9001, but has a few more stipulations to help
Subscribe to our Newsletter for the latest news, updates and offers.




Star Rapid Manufacturing, 2017. All Rights Reserved.